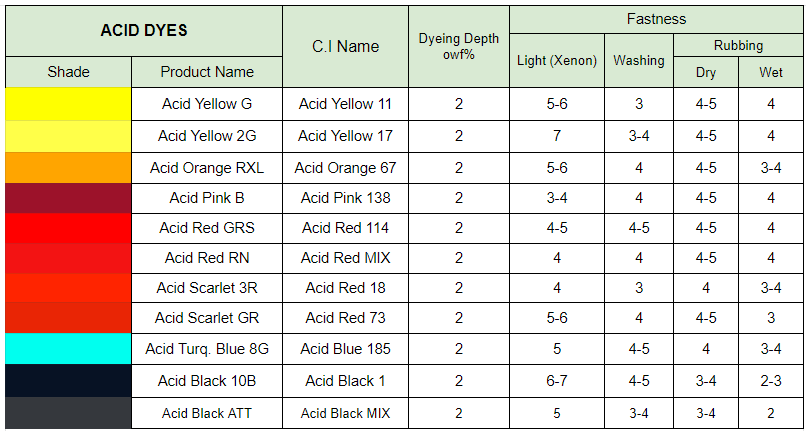
Acid dyes which are anionic, water soluble and normally applied from acidic bath. Acid dyes are applied to the fibre under acid conditions. Acid dyes have very good shade range and standard fastness properties. Wet fastness of acid dyes can be improved after-treating the dyeing in some cases. Compatibility within combination is important as blocking effects and differential coverage of yarn irregularities may result if selection is not carefully done. Acid dyes fix to the fibre by hydrogen bonding, vander waals forces and ionic linkages. There are 3 kinds of acid dyes such as neutral acid dyes, weak acid dyes, strong acid dyes.
Acid dyes are normally used for dyeing natural protein like wool and silk. It's also used for dyeing synthetic polyamide like nylon and sometimes acrylic.
- Acid dye – x %
- Acetic acid – 2 – 4 %
- Glablur salt – 5 – 10 %
- pH – 4 – 5
In this dyeing process, first need to set the dye bath at 40 to 45 °C for dyeing. Then introduce the mixture of acetic acid + Glauber salt in the dye bath and introduce dye solution . The temperature of the dye bath should be 40 to 45°C and then continue the dyeing for 45 minute and then raise the temperature to 100°C for 30 – 90 minute after complete dyeing. Then cool – darin – wash – and dry.